What is AUCDI?
The Australian Core Data for Interoperability (AUCDI) is a colection of data groups and data models representing the clinical requirements for data entry, data use and sharing of clinical information supporting patient care.
AUCDI provides the common data foundation that can be reused across multiple common use cases. Some use cases, such as the Patient Summary (PS) and Chronic Condition Management (CCM), will be fully encapsulated within AUCDI as it develops.
Future use-case specific data sets may be developed using the same approach, potentially reusing components from AUCDI, AUeReqDI, and other specifications as they evolve.
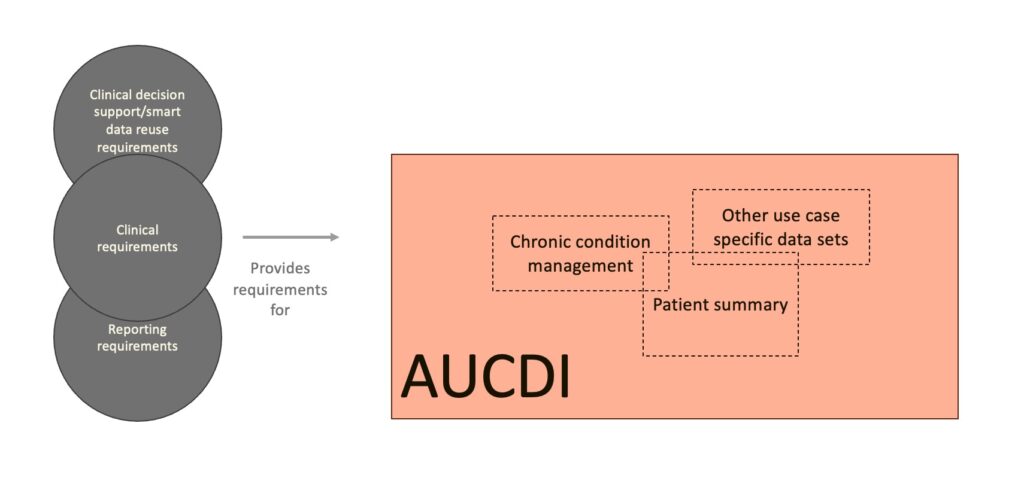
AUCDI as a foundation for use case specific data sets.
The AUCDI data groups are comprised of two components – clinical information models and terminologies.
Clinical information models
An information model is a technical term commonly used in software engineering to describe the representation of data semantics. It is like a blueprint or map of how information and its meaning and relationships are managed and organised within a system.
Each clinical information model describes a single, discrete clinical concept and its clinically agreed data structure, pattern, and content. Some information models will be simple; others will represent a more complex grouping of related data.
Within this document, clinical information models are referred to as ‘data groups’. Examples include:
- ‘Adverse reaction risk summary’,
- ‘Procedure completed event’, and
- ‘Blood Pressure’.
Each information model includes:
- Metadata descriptions about the clinical concept and its intended purpose, use, and misuse,
- One or more component data elements, each associated with attributes such as data types, recommended values, and constraints, and
- Relationships between data elements.
Each data group is designed to be reused across many data sets, projects, clinical scenarios, and geographical locations. Each one will vary in detail, growing in granularity over time as requirements for applications in different contexts are further understood.
This core clinical data is living artefact, designed to grow over time as changes in clinical information requirements change. The intent is to have a foundational master set of data groups inclusive of all relevant data elements and their attributes.
Existing projects and priorities informed the development of these models and intended to be agnostic to any use case, project, application, or intent.
As projects and clinical systems increasingly use the same data groups, interoperability becomes significantly more straightforward because of these shared information models, minimising the need for data transformation and mapping, which can lead to errors or data loss.
Terminologies, SNOMED CT-AU and value sets
Within each information model, data can be captured as text through free text narrative or by using structured, coded text which involves selecting words or phrases from a controlled terminology. A standardised set of these terms is known as a value set. Selection from an agreed value set from a standardised clinical terminology can dramatically improve the quality and consistency of data recording. Benefits include correct spelling, acceptable synonyms and limiting data entry selection to clinically appropriate values based on the context. Reusing agreed and validated terminology value sets within the clinical information models further enhances data exchange, clinical decision support, querying, and analysis of health data.
In Australia, SNOMED CT-AU has been adopted as the standard for recording structured clinical data in health records. In the Sparked program, SNOMED CT-AU is the primary terminology from which value sets are derived to support standardised coded data entry in the AUCDI’s clinical information models. Other code systems, such as LOINC, may also be used where agreed through consultation with the community of practice. Standardised terminologies have been selected because they are internationally recognised with an existing global implementation footprint.
Many SNOMED CT-AU value sets have already been developed and published by the National Clinical Terminology Service (NCTS). These nationally agreed and published value sets are maximal in nature to support reuse across multiple use cases and support the breadth of the ecosystem to enable interoperability. Where the clinical context or use case requires it, specific IG specification or vendor implementations may specify constrained subsets of the AUCDI value sets.
