AUCDI Release 1
The final version of AUCDI Release 1 was published on June 25, 2024.
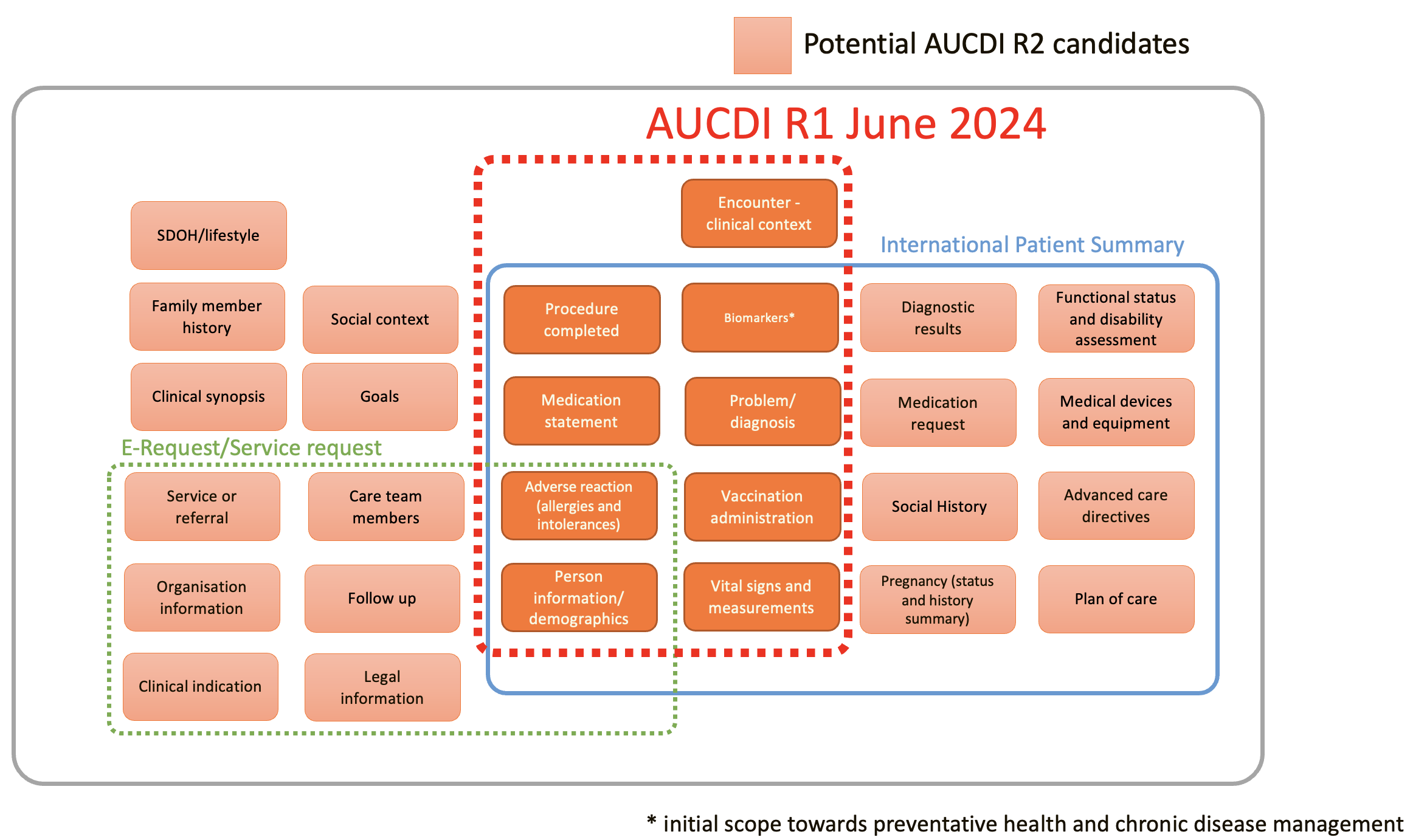
Scope of AUCDI Release 1
The R1 scope of AUCDI includes:
- Adverse reaction risk summary (allergies and intolerances),
- Problem/Diagnosis summary,
- Procedure completed event,
- Vaccination administration event (immunisations),
- Vital signs, measurements and other biomarkers for chronic disease and preventative health with an initial scope of cardiovascular risk calculation and diabetes care,
- Medication use statement,
- Sex and gender, and
- Encounter information necessary to provide clinical context.
The focus of the AUCDI is the representation of the clinical content necessary for each of the data groups.
Unless it is of clinical significance and requires clinical validation, the R1 scope of AUCDI does not include:
- Patient (including date of birth, Aboriginal and Torres Strait Islander status),
- System information, or system-derived information – includes information related to technical aspects of recording data (such as author and record date/timestamp) and will be managed in the technical implementation specifications (for example in a FHIR IG),
- Administrative, workflow and billing information,
- User interface or form implementation requirements,
- Higher-level technical concepts such as security, access, privacy, and consent, and
- Non-clinical recording context such as author, location of service.
Each data group is designed as a standalone module. The data groups can be mixed and matched to represent larger clinical data sets for use in different contexts.